k electron affinity|Lesson Explainer: Electron Affinity : Bacolod Ago 11, 2023 Kamii Roy photos & videos. EroMe is the best place to share your erotic pics and porn videos. Every day, thousands of people use EroMe to enjoy free photos and videos. . lana roy arpa roy anaya roy roy roy anaya kamii writtima roy mouni roy. FAQ - Terms - DMCA/Abuse - Creator/Paysite - Feedback - Explore. Please login or register. OK.
k electron affinity,Ago 11, 2023
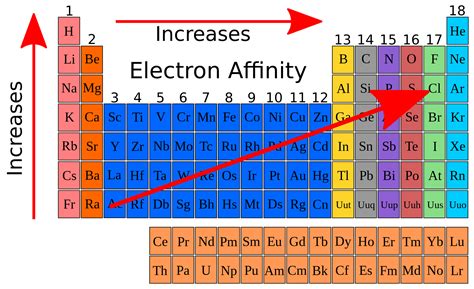
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to .

This page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here.k electron affinityThis page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here.

The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.k electron affinity Lesson Explainer: Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom .Chemists define electron affinity as the change in energy, measured in units of kJ/mole, experienced when an electron is added to a gaseous atom. This process creates a negative ion. This process differs from . About. Transcript. Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an .Electron affinity is defined as. The amount of energy released when an electron is added to a neutral atom to form an anion. The electron affinity is the potential energy change of the atom when an electron is added to .
k electron affinity|Lesson Explainer: Electron Affinity
PH0 · electron affinity
PH1 · What is Electron Affinity?
PH2 · What Is Electron Affinity?
PH3 · Lesson Explainer: Electron Affinity
PH4 · Electron affinity: period trend (video)
PH5 · Electron affinity (data page)
PH6 · Electron affinity
PH7 · Electron Affinity Chart (Labeled Periodic table + List)
PH8 · Electron Affinity
PH9 · 7.5: Electron Affinities